Position on REACH RESTRICTION on intentionally added microplastics
EDANA, the voice of the nonwovens and related industries, represents companies which supply products and services ranging from raw materials to finished products (including Absorbent Hygiene Products – AHP) and everything in between including machinery, components, and development and testing facilities. It represents not only all types of nonwovens, but also materials often used together with nonwovens, such as films and superabsorbent polymers (SAP).
With this document, EDANA supports the nonwovens and related industries by providing an overview of the application of the REACH restriction on intentionally added microplastics1 to economic operators who handle:
- Superabsorbent polymers (SAP)
- Absorbent Hygiene Products (AHP) containing SAP
- Nonwovens fibres & nonwovens fabrics
- Raw materials used to produce nonwovens fibres and nonwovens fabrics
EDANA and its Member Companies are committed to ensuring the safety of consumers while prioritising environmental responsibility. As such, EDANA welcomes the European Commission's efforts to address microplastics in the environment and appreciate the recognition that the risk from releases of SAP is minimised, when used in products like AHP².
EDANA continues to demonstrate a strong commitment to EU and national regulatory compliance, maintaining rigorous standards of safety and efficacy, while providing clear evidence that SAP which are contained in AHP products do not contribute to environmental microplastic pollution.
Superabsorbent polymers (SAP)
In this document, the term SAP applies to the class of polymers which are formed by the cross linking of long, repeating chains of acrylic acid salts, normally sodium acrylate.
SAP are microplastics according to the definition in REACH.
Placing them on the market is banned, unless either:
- They are for use at industrial sites
- They are contained by technical means so that releases to the environment are prevented when used inaccordance with the instructions for use during the intended end use
- The physical properties of SAP are permanently modified during intended end use in such a way that thepolymer no longer falls within the scope of the restriction
- They are permanently incorporated into a solid matrix during intended end use
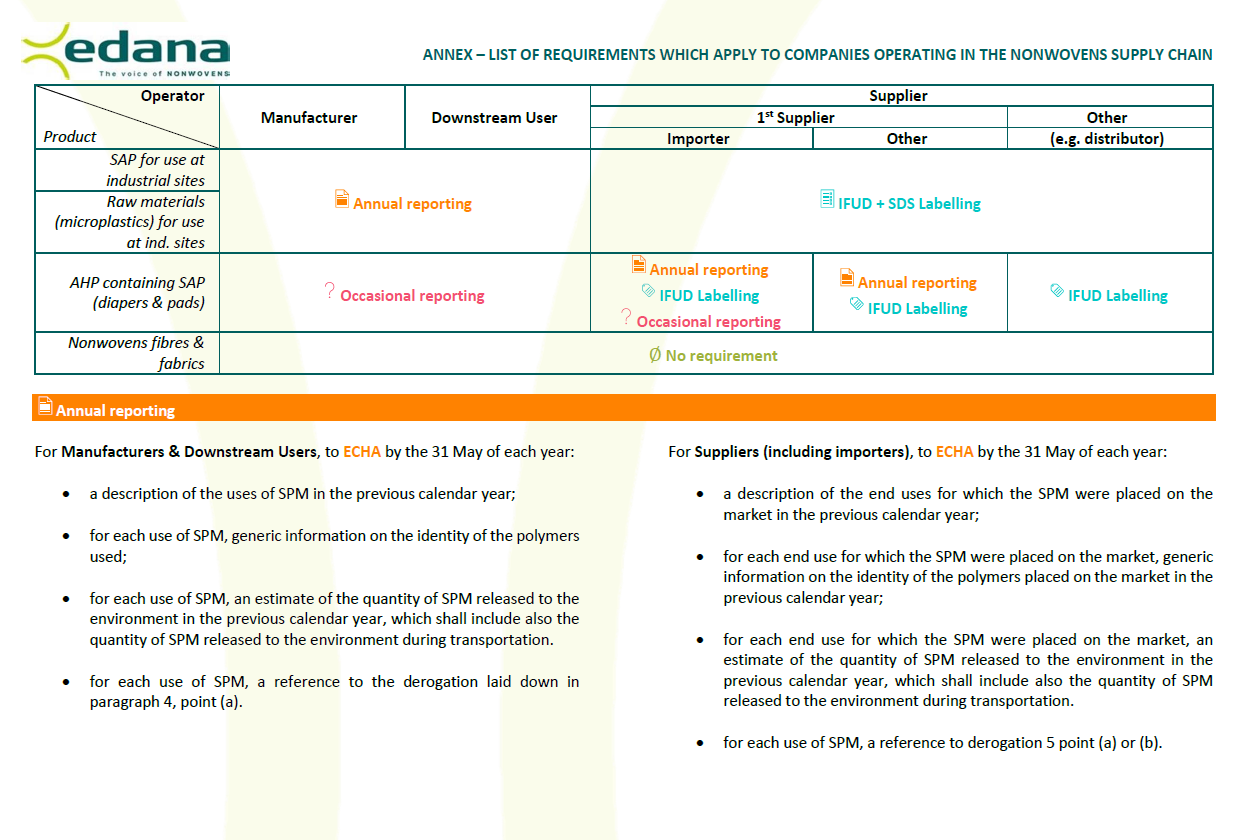
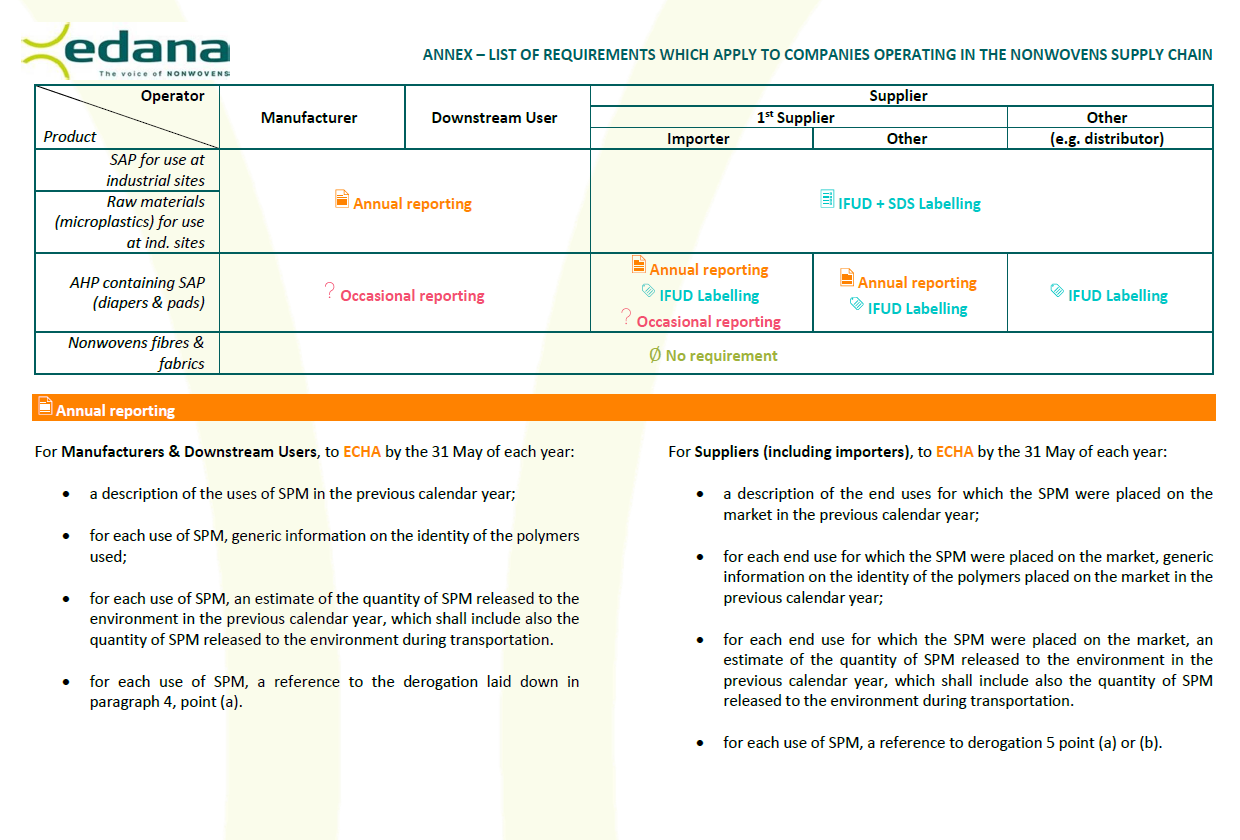
And the operator complies with the relevant labelling and reporting requirements (see Annex).
Absorbent Hygiene Products (AHP) containing SAP
AHP containing SAP are diapers and pads.
The European Commission published an explanatory guide³ that explicitly highlights the derogations applicable to SAP used in AHP.
“Paragraph 5 [of the REACH restriction] derogates SPM for which the release to the environment can be minimised during use, because the SPM are either:
- contained by technical means so that releases to the environment are prevented when used in accordance with the instructions for use during the intended end use (Paragraph 5(a)); E.g: SPM in chromatography columns, toners, water-filtering cartridges, diapers, incontinence pads or menstrual pads.
- Permanently modified during intended end use so that they stop being SPM (Paragraph 5(b)); Eg: swellable polymers in diapers and other applications; film-forming polymers in e.g. cosmetic products, detergents and maintenance products, paints and coatings.
- […]”
Therefore, placing AHP containing SAP on the market is derogated, providing that the operator can comply with either or both of the following requirements:
- SAP are contained in the AHP by technical means so that releases to the environment are prevented when used in accordance with the instructions for use during the intended end use
- The physical properties of SAP are permanently modified during intended end use in such a way that the polymer no longer falls within the scope of the restriction
And the operator complies with the relevant labelling and reporting requirements (see Annex).
According to Question 7.5 of Part II of the European Commission’s explanatory guide on the REACH restriction of SPM4: “Swellable solid polymers that fulfil the SPM conditions […] are within the scope of the restriction. However, the derogations under Paragraph 5 may apply to swellable SPM.
For example, swellable SPM contained in diapers may fall under Paragraph 5(a).
Paragraph 5(b) applies to swellable SPM that, during intended end use, become larger than 5 mm in at least one dimension and remain larger during the intended end use of the product. For example, in the case of diapers, Paragraph 5(b) would apply if the polymers are still swollen when the diaper is removed and disposed of. See also Text Box 9 in Section 6, Part I of [the] Explanatory Guide.
[…]
Paragraph 5(b) also applies to swellable SPM that lose their solid state, or stop being in particles, during intended end use”.
Nonwovens fibres & nonwovens fabrics
Nonwovens fibres and fabrics are articles under REACH5: the restriction does not apply to these products6.
Operators do not need to comply with any of the requirements of the restriction on microplastics.
Raw materials used to produce nonwovens fibres and nonwovens fabrics
If raw materials match the criteria of the definition of synthetic polymer microparticles (i.e. microplastics), they are banned, unless they are placed on the market for use at industrial sites providing that the operator complies with the relevant labelling and reporting requirements (see Annex).
Operators can rely on the European Commission’s explanatory guide7 to determine whether an object matches the criteria of the definition of microplastics.
___
1. Commission Regulation (EU) 2023/2055 of 25 September 2023 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards synthetic polymer microparticles (link)
2. Commission Regulation (EU) 2023/2055, Recital 18, and Explanatory Guide | REACH restriction of synthetic polymer microparticles | Version 1, 2025 (link)
3. Explanatory Guide | REACH restriction of synthetic polymer microparticles | Version 1, 2025 (link)
4. Explanatory Guide | REACH restriction of synthetic polymer microparticles | Version 1, 2025 (link)
5. ECHA Guidance on requirements for substances in articles, p. 83
6. Explanatory Guide | REACH restriction of synthetic polymer microparticles | Version 1, 2025 (link)
7. Explanatory Guide | REACH restriction of synthetic polymer microparticles | Version 1, 2025 (link)
ANNEX :